Your How to work out concentration from absorbance images are available. How to work out concentration from absorbance are a topic that is being searched for and liked by netizens today. You can Find and Download the How to work out concentration from absorbance files here. Download all royalty-free photos and vectors.
If you’re looking for how to work out concentration from absorbance images information related to the how to work out concentration from absorbance topic, you have pay a visit to the ideal blog. Our site always provides you with hints for downloading the maximum quality video and image content, please kindly search and find more informative video content and graphics that match your interests.
How To Work Out Concentration From Absorbance. The concentration of a sample can be calculated from its absorbance using the BeerLambert law which is expressed as follows. Use the following formula to estimate protein concentration. Using the standard curve below calculate the concentration of an unknown solution if its absorbance is 055. We also use third-party cookies that help us analyze and understand how you use this website.
 Chem 125 Experiment Ii From websites.umich.edu
Chem 125 Experiment Ii From websites.umich.edu
Molar absorptivity compensates for this by dividing by both the concentration and the length of the solution that the light passes through. Law is expressed as Absorbance eL c. A ε c p Where ε is the molar absorptivity or molar extinction coefficient in L mol -1 cm -1 c is the concentration of the solute in solution in molL. Absorbance 2 logT Transmittance T is the fraction of incident light which is transmitted. How do you calculate absorbance from absorption coefficient. Using the standard curve below calculate the concentration of an unknown solution if its absorbance is 055.
Absorbance is calculated from the negative decadic logarithm of transmission.
You also have the option to opt-out of these. Molar absorptivity compensates for this by dividing by both the concentration and the length of the solution that the light passes through. The proportionality constant of the equation is termed as the molar extinction coefficient of the substance. In the equation for a straight line y mx b m is the slope of the line. Absorbance 2 logT Transmittance T is the fraction of incident light which is transmitted. Absorbance can be calculated from percent transmittance T using this formula.
 Source: handling-solutions.eppendorf.com
Source: handling-solutions.eppendorf.com
Beers Law AEbc helped to develop the linear equation since absorbance was equal to y Eb was equal to m and the concentration c was equal to the slope x in the equation ymxb. The concentration of a sample can be calculated from its absorbance using the BeerLambert law which is expressed as follows. Absorbance can be calculated from percent transmittance T using this formula. Beers Law AEbc helped to develop the linear equation since absorbance was equal to y Eb was equal to m and the concentration c was equal to the slope x in the equation ymxb. That means that you can then make comparisons between one.
 Source: khanacademy.org
Source: khanacademy.org
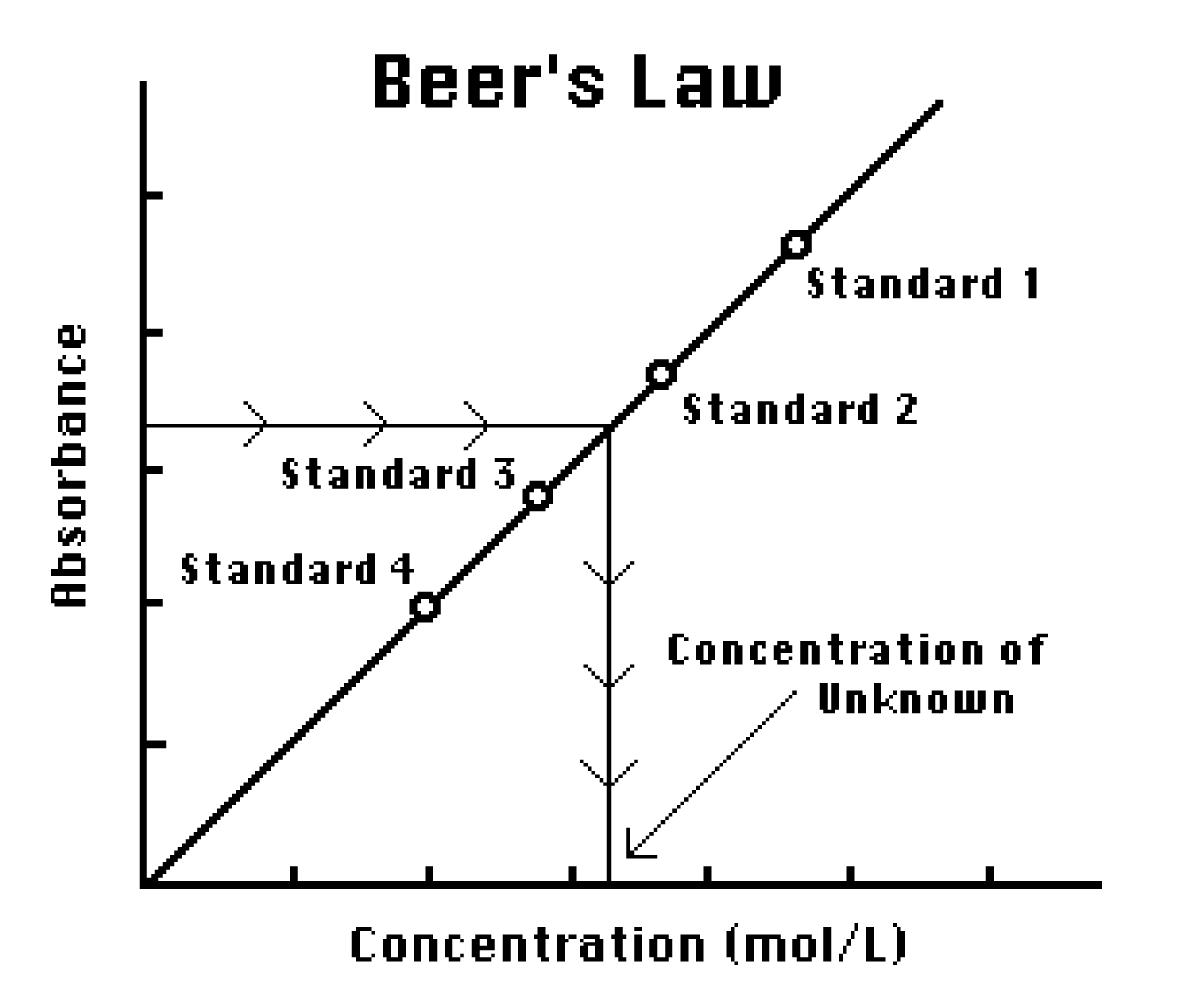
The sample concentration will be obtained from the standard curve since the OD value of the sample corresponds to a concentration value on the x-axis. Unknown mgml 50 mgml x Measured A260 x dilution factor see below Concentration determinationthe instructor will help in the. According to the Beer Lambert Law the Absorbance is proportional to the path length distance that light travels through the material and the concentration of the material. Absorbance is calculated from the negative decadic logarithm of transmission. The sample concentration will be obtained from the standard curve since the OD value of the sample corresponds to a concentration value on the x-axis.
 Source: people.hws.edu
Source: people.hws.edu
Concentration is in mgml or molarity depending on which type coefficient is used. Where y is the absorbance m is the slope and x is the concentration. Specifically the amino acids tyrosine and tryptophan have. Absorbance can be calculated from percent transmittance T using this formula. Essentially it works out a value for what the absorbance would be under a standard set of conditions - the light traveling 1 cm through a solution of 1 mol dm-3.
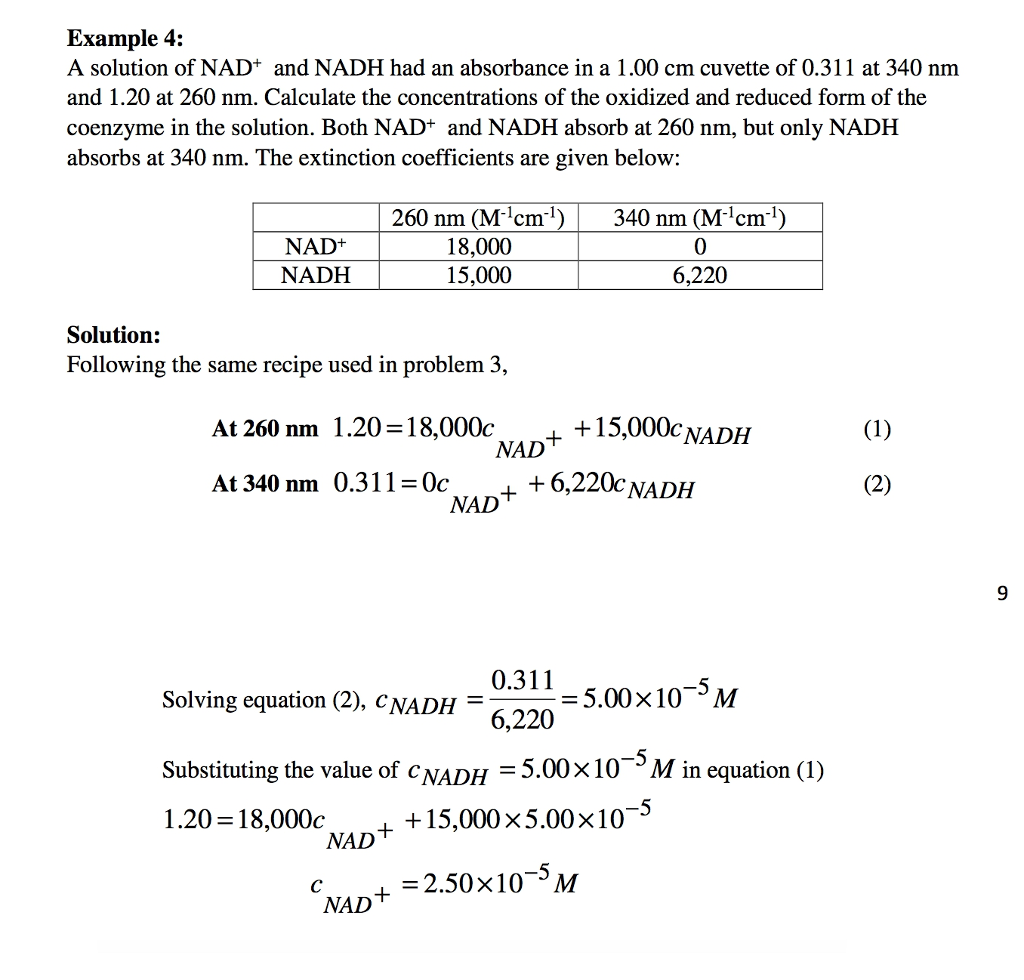 Source: chegg.com
Source: chegg.com
Unknowns with possible nucleic acid contamination. Concentration mgml Absorbance at 280 nm divided by path length cm Pure protein of known absorbance coefficient. From the slope of the best-fit line together with the absorbance you can now calculate the concentration. Once you have that you can compare the absorbance value of an unknown sample to figure out its concentration. In the equation for a straight line y mx b m is the slope of the line.
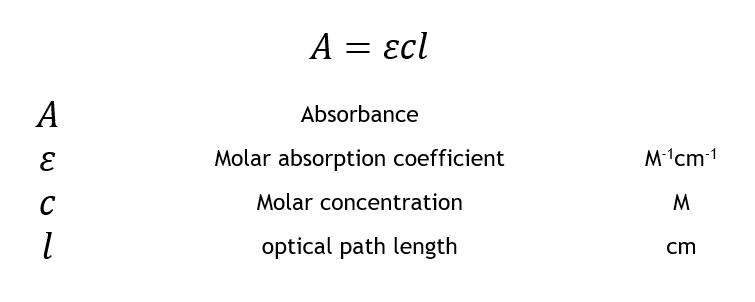 Source: edinst.com
Source: edinst.com
A mCl Aabsorbance m molar extinction coefficient C concentration lpath length of 1 cm. MMacvolCoursesBiol 114F03LabLab2speclab2writeup03doc - 3 - It is also possible to calculate the concentration if we know the slope of the standard curve. Essentially it works out a value for what the absorbance would be under a standard set of conditions - the light traveling 1 cm through a solution of 1 mol dm-3. Absorbance 2 logT Transmittance T is the fraction of incident light which is transmitted. From the slope of the best-fit line together with the absorbance you can now calculate the concentration.
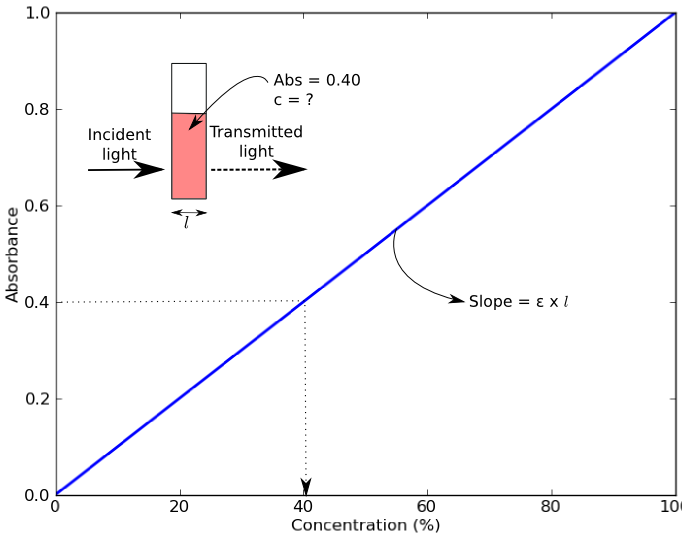 Source: stuff.iorodeo.com
Source: stuff.iorodeo.com
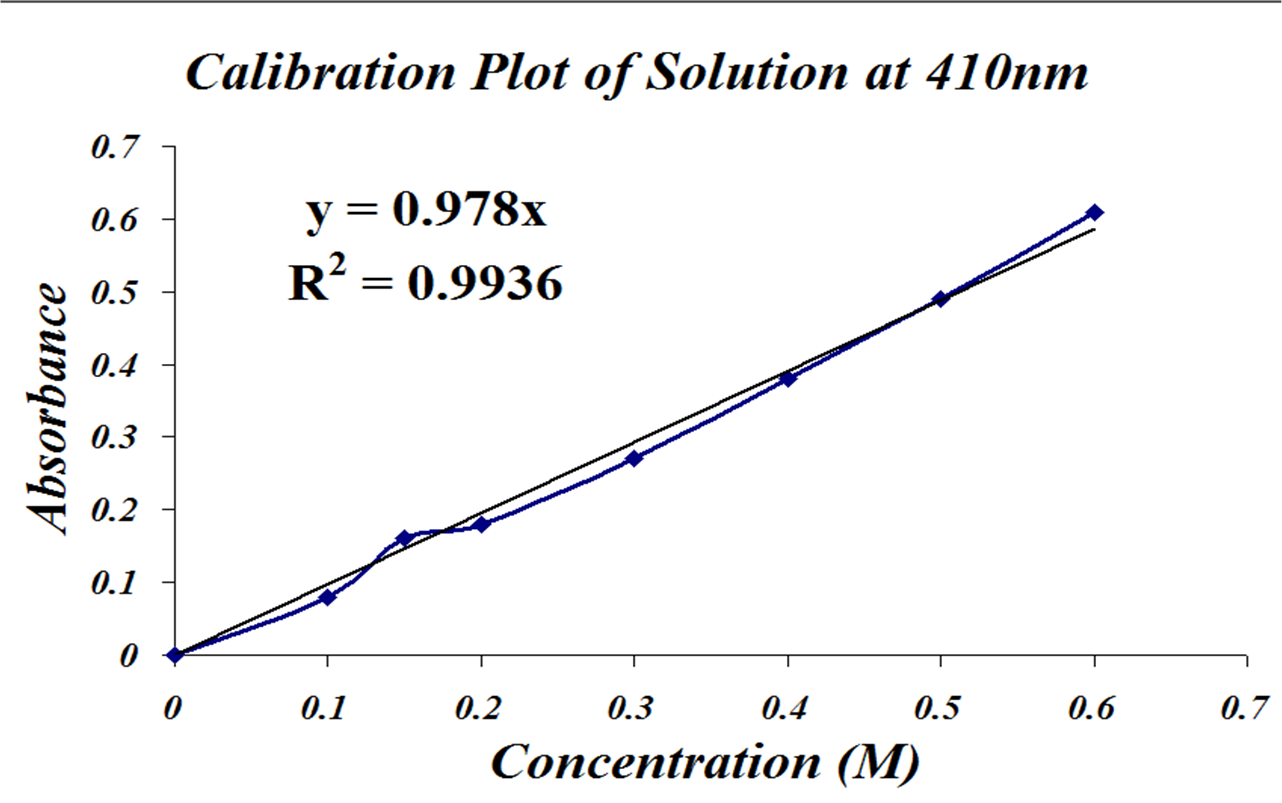
Using the standard curve below calculate the concentration of an unknown solution if its absorbance is 055. MMacvolCoursesBiol 114F03LabLab2speclab2writeup03doc - 3 - It is also possible to calculate the concentration if we know the slope of the standard curve. Using a serial dilution describe how you would prepare 10 mL of a 1 01 and 001 solution of NaOH. Concentration is in mgml or molarity depending on which type coefficient is used. First of all you should made standard curve concentration against absorbance.
 Source: researchgate.net
Source: researchgate.net
Thus x y m. The concentration of a sample can be calculated from its absorbance using the BeerLambert law which is expressed as follows. Absorbance A C x L x Ɛ Concentration C A L x Ɛ. The proportionality constant of the equation is termed as the molar extinction coefficient of the substance. Using the standard curve below calculate the concentration of an unknown solution if its absorbance is 055.
 Source: websites.umich.edu
Source: websites.umich.edu
Specifically the amino acids tyrosine and tryptophan have. In the equation for a straight line y mx b m is the slope of the line. That means that you can then make comparisons between one. Use the following formula to estimate protein concentration. Absorbance A C x L x Ɛ Concentration C A L x Ɛ.
 Source: study.com
Source: study.com
The stock solution of NaOH is 10. Using a serial dilution describe how you would prepare 10 mL of a 1 01 and 001 solution of NaOH. Such subtraction is often automatic in the ELISA software. The sample concentration will be obtained from the standard curve since the OD value of the sample corresponds to a concentration value on the x-axis. Specifically the amino acids tyrosine and tryptophan have.
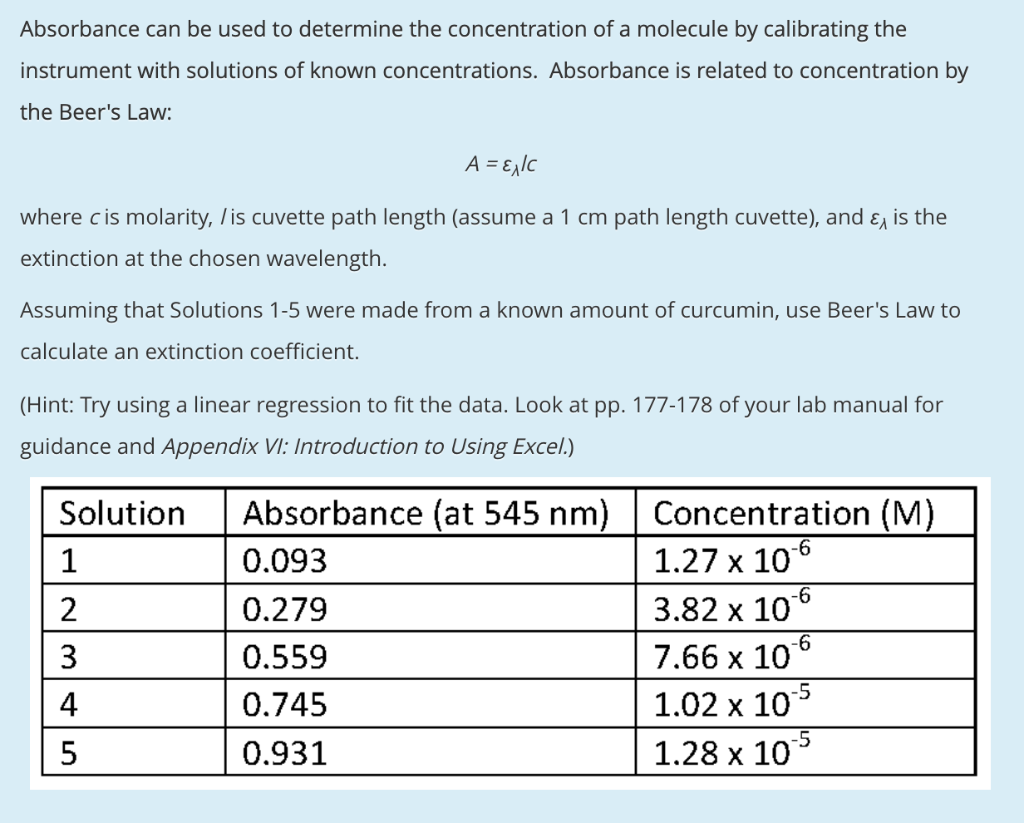 Source: chegg.com
Source: chegg.com
The absorbance of the sample is divided by the value of the slope and the concentration is. The stock solution of NaOH is 10. Absorbance 2 logT Transmittance T is the fraction of incident light which is transmitted. The equation for Beers Law also describes a straight line with an. Using the standard curve below calculate the concentration of an unknown solution if its absorbance is 055.
 Source: harmslab.uoregon.edu
Source: harmslab.uoregon.edu
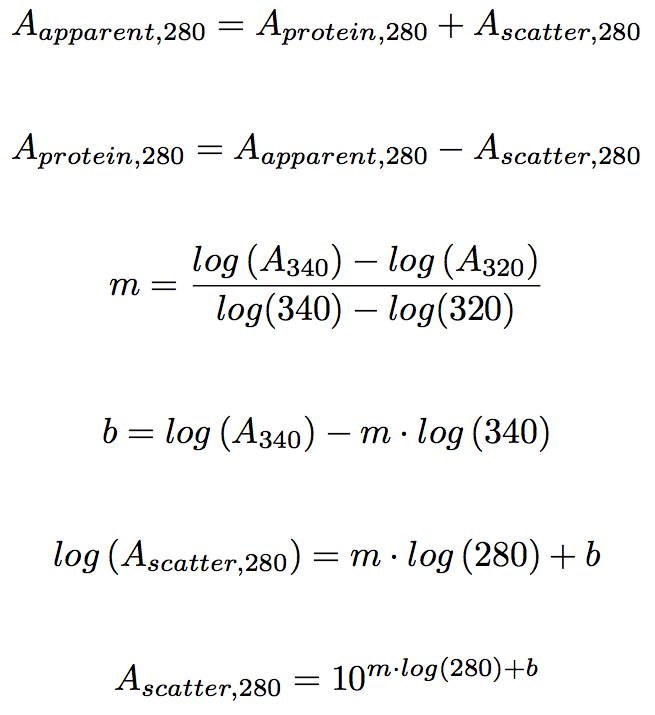
Draw diagram as part of your description. A mCl Aabsorbance m molar extinction coefficient C concentration lpath length of 1 cm. The mean absorbance value of the blank wells is recommended to be subtracted from the other values prior to creating the standard curve. Between absorbance and DNA concentration we can use some simple algebra and reformulate as follows. Molar absorptivity compensates for this by dividing by both the concentration and the length of the solution that the light passes through.
 Source: edinst.com
Source: edinst.com
Such subtraction is often automatic in the ELISA software. Concentration Absorbance at 280 nm divided by absorbance coefficient. Draw diagram as part of your description. A ε c p Where ε is the molar absorptivity or molar extinction coefficient in L mol -1 cm -1 c is the concentration of the solute in solution in molL. Concentration is in mgml or molarity depending on which type coefficient is used.
 Source: researchgate.net
Source: researchgate.net
Absorbance can be calculated from percent transmittance T using this formula. Between absorbance and DNA concentration we can use some simple algebra and reformulate as follows. Once you have that you can compare the absorbance value of an unknown sample to figure out its concentration. Using the standard curve below calculate the concentration of an unknown solution if its absorbance is 055. In the equation for a straight line y mx b m is the slope of the line.

Absorbance can be calculated from percent transmittance T using this formula. In other words its the amount of light that successfully passes through the substance and comes out the other side. Absorbance 2 logT Transmittance T is the fraction of incident light which is transmitted. In the equation for a straight line y mx b m is the slope of the line. According to the Beer Lambert Law the Absorbance is proportional to the path length distance that light travels through the material and the concentration of the material.
 Source: researchgate.net
Source: researchgate.net
Use the following formula for a path length of 1 cm. Specifically the amino acids tyrosine and tryptophan have. Why is absorbance at 280 nm used for protein determination. Use the following formula for a path length of 1 cm. Use the following formula to estimate protein concentration.
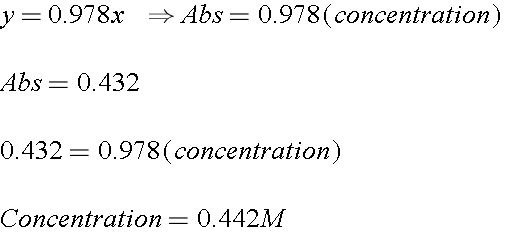 Source: websites.umich.edu
Source: websites.umich.edu
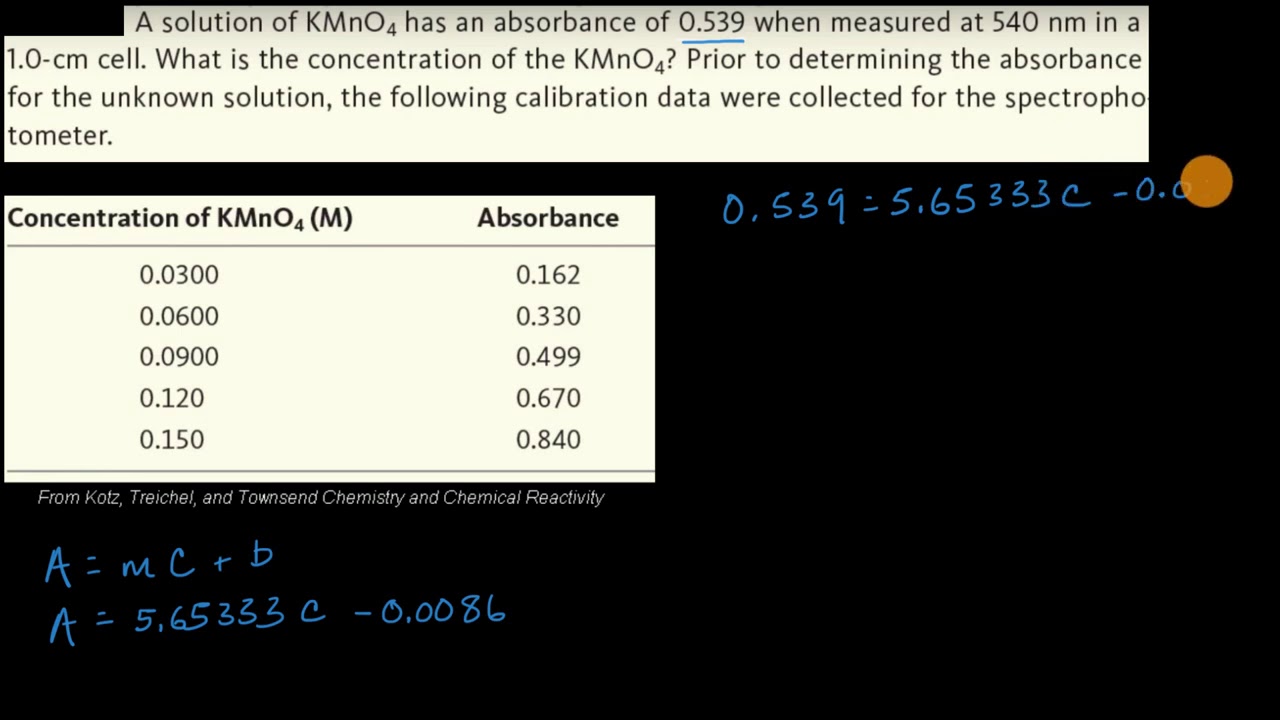
To find the concentration for a solution that has an absorbance of 060 you will first need to find the slope of the BEST-FIT line. To find the concentration for a solution that has an absorbance of 060 you will first need to find the slope of the BEST-FIT line. Out of these cookies the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. The absorbance of the sample is divided by the value of the slope and the concentration is. Draw diagram as part of your description.
 Source: vernier.com
Source: vernier.com
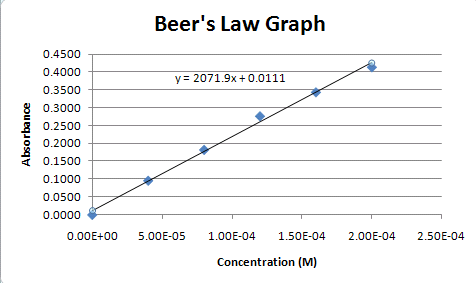
The linear relationship between absorbance and concentration displays that absorbance depends on the concentration. How do you calculate absorbance from absorption coefficient. Absorbance can be calculated from percent transmittance T using this formula. The sample concentration will be obtained from the standard curve since the OD value of the sample corresponds to a concentration value on the x-axis. We also use third-party cookies that help us analyze and understand how you use this website.
 Source: study.com
Source: study.com
These cookies will be stored in your browser only with your consent. Once you have that you can compare the absorbance value of an unknown sample to figure out its concentration. Concentration Absorbance at 280 nm divided by absorbance coefficient. That means that you can then make comparisons between one. Unknown mgml 50 mgml x Measured A260 x dilution factor see below Concentration determinationthe instructor will help in the.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to work out concentration from absorbance by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.