Your How does ir spectroscopy work images are available. How does ir spectroscopy work are a topic that is being searched for and liked by netizens now. You can Download the How does ir spectroscopy work files here. Get all free photos and vectors.
If you’re looking for how does ir spectroscopy work images information connected with to the how does ir spectroscopy work keyword, you have visit the right site. Our website always gives you suggestions for seeking the maximum quality video and image content, please kindly hunt and find more enlightening video content and images that fit your interests.
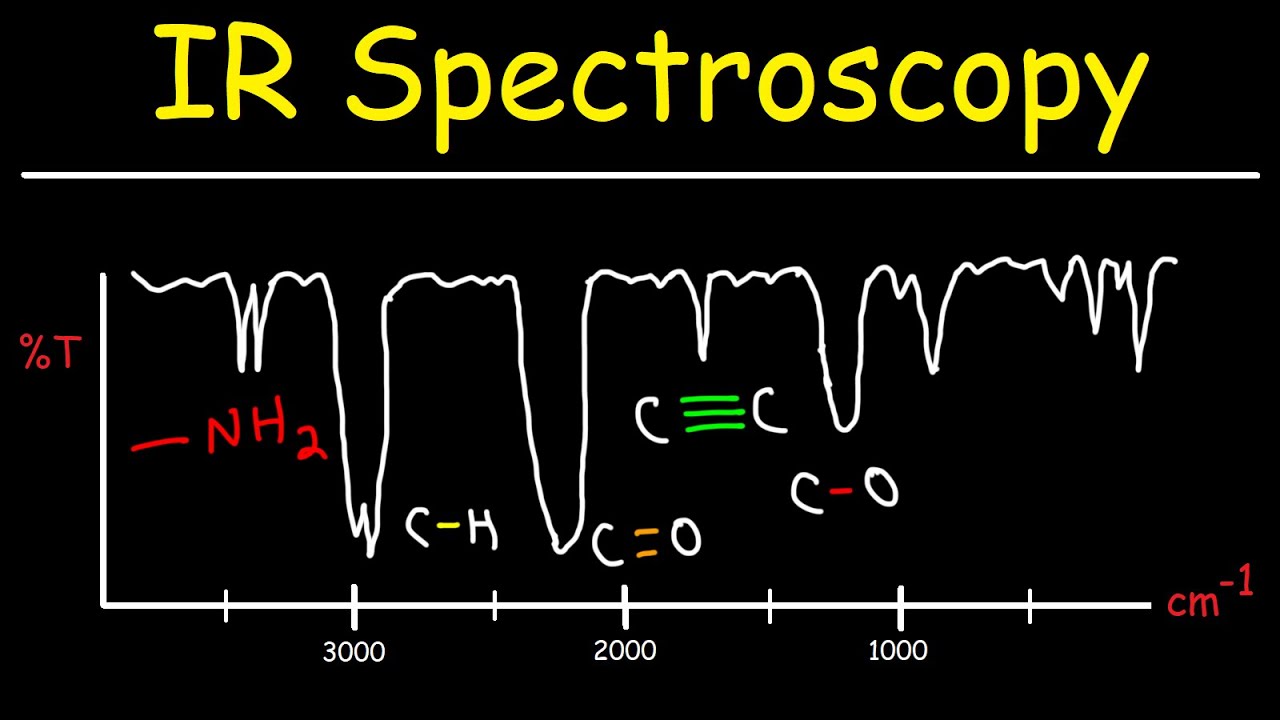
How Does Ir Spectroscopy Work. Easy to use - normal operation consists of loading a sample cell and starting the instrument. No hazardous chemical waste - no chemicals are used at all. Infrared IR light is a wavelength of energy that is invisible to the human eye. It is used by chemists to determine functional groups in molecules.
 Pin On Studystudystudy From pinterest.com
Pin On Studystudystudy From pinterest.com
Molecules tend to absorb these. What is IR Spectroscopy. Infrared IR spectroscopy is a vibrational spectroscopic technique based on the absorption of infrared radiation by matters that excite vibrations of molecular bonds. Some of the infrared radiation is absorbed by the sample and some of the infrared radiation is transmitted through the sample. Infrared IR light is a wavelength of energy that is invisible to the human eye. Basic schematic of Michelson interferometer courtesy of Leng Materials Characterization The most important component of FTIR spectroscopy and where it derives its distinction from typical infrared spectroscopy is the Michelson interferometer.
Basic schematic of Michelson interferometer courtesy of Leng Materials Characterization The most important component of FTIR spectroscopy and where it derives its distinction from typical infrared spectroscopy is the Michelson interferometer.
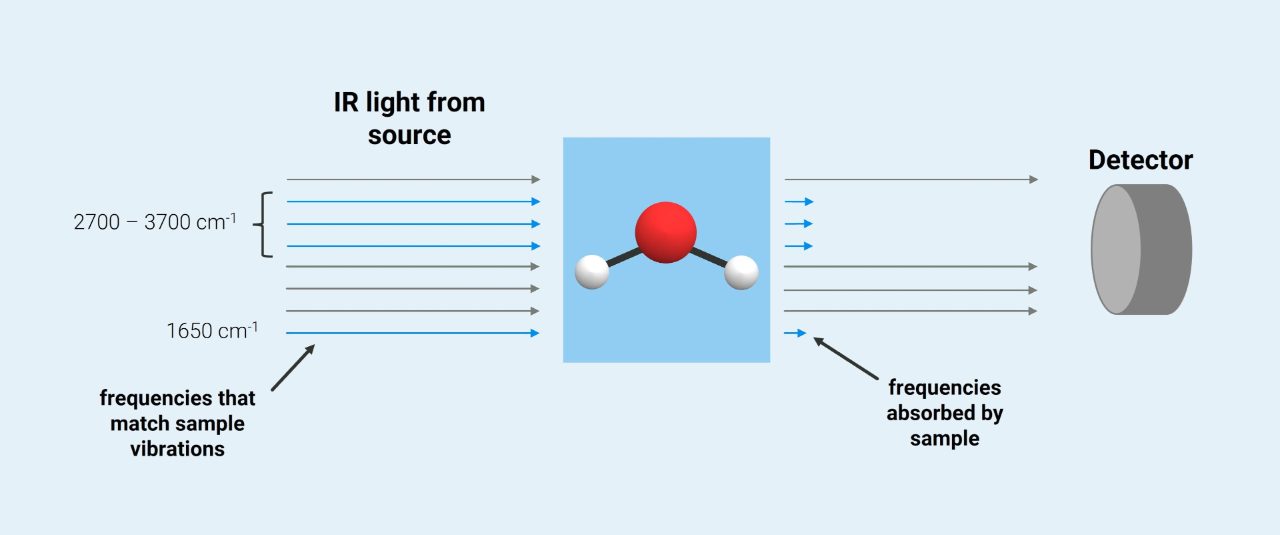
This spectrometer can also measure the number of absorbing molecules. Frequency or wavelength on the X-axis. Near Infrared NIR spectroscopy uses light transmission and absorption to measure various constituents in a sample material such as. Those peaks represent the wavelengths of infrared light that dont get to the detector because theres so. How does an IR Spectrometer work. This spectrometer can also measure the number of absorbing molecules.
 Source: researchgate.net
Source: researchgate.net
The higher the concentration of a compound the more infrared light is absorbed and less is reflected to the NIR instrument. Near Infrared NIR spectroscopy uses light transmission and absorption to measure various constituents in a sample material such as. In the interferometer the light passes through a beam splitter which sends the. Infrared Spectroscopy is the analysis of infrared light interacting with a molecule. IR Spectroscopy detects frequencies of infrared light that are absorbed by a molecule.
 Source: pinterest.com
Source: pinterest.com
Infra-red Spectroscopy Infra-red spectroscopy is used by organic chemists to help them identify compounds. In every scan all source radiation goes through the sample. Objects can have their relative temperatures measured by how much of this energy they give off. The most common source of this energy is heat. Ad Near-infrared Spectroscopy NIR Solutions From Malvern Panalytical.
 Source: jasco-global.com
Source: jasco-global.com
No hazardous chemical waste - no chemicals are used at all. In infrared spectroscopy analysis infrared radiation is transmitted through a sample. Heres a short table of common absorption frequencies. Moisture starch protein fat and oils. This spectrometer can also measure the number of absorbing molecules.
 Source: bruker.com
Source: bruker.com
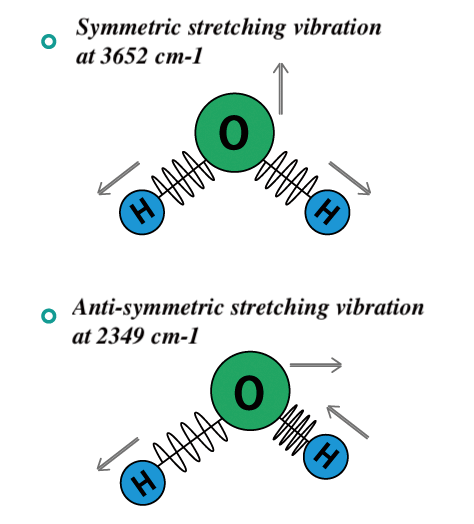
This spectrometer can also measure the number of absorbing molecules. Infrared IR light is a wavelength of energy that is invisible to the human eye. How does an IR Spectrometer work. Infrared Spectroscopy IR Triggering molecular vibrations through irradiation with infrared light. Strong bonds vibrate at a higher frequency faster and heavier atoms make the bond vibrate at a lower frequency slower.
 Source: youtube.com
Source: youtube.com
The higher the concentration of a compound the more infrared light is absorbed and less is reflected to the NIR instrument. Some of the infrared radiation is absorbed by the sample and some of the infrared radiation is transmitted through the sample. Provides information about molecular mass and atom connectivity. The spectrometer detects this absorption and records it as a peak in a plot of transmission versus frequency. Objects can have their relative temperatures measured by how much of this energy they give off.
 Source: pinterest.com
Source: pinterest.com
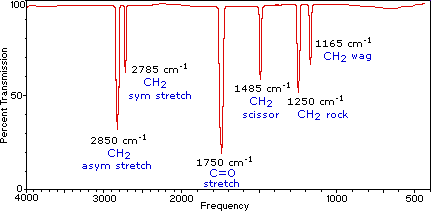
The main use of this technique is in organic and inorganic chemistry. Strong bonds vibrate at a higher frequency faster and heavier atoms make the bond vibrate at a lower frequency slower. Whereas in dispersive IR spectroscopy monochromatic light. What are all these squiggles. Different types of bonds respond to the IR radiation differently.
 Source: socratic.org
Source: socratic.org
When the sample is exposed to infrared wavelengths the vibrations are measured at different frequencies. What are all these squiggles. Infrared spectrometers sometimes referred to as IR spectrometers measure vibrations in the interatomic bonds within the sample being tested. This spectrometer can also measure the number of absorbing molecules. Infrared IR sometimes called infrared light is electromagnetic radiation EMR with wavelengths longer than those of visible lightIt is therefore invisible to the human eye.
 Source: sciencedirect.com
Source: sciencedirect.com
How does an IR Spectrometer work. In infrared spectroscopy analysis infrared radiation is transmitted through a sample. The spectrometer works by sending source energy through an interferometer and through the sample. The interferometer is a fundamentally different piece of equipment than the monochromator found in UVVis spectrometers. Mass spectrometry Bombardment of the sample with electrons and detection of resulting molecular fragments.
 Source: cz.pinterest.com
Source: cz.pinterest.com
Strong bonds vibrate at a higher frequency faster and heavier atoms make the bond vibrate at a lower frequency slower. Different types of bonds respond to the IR radiation differently. Some of the infrared radiation is absorbed by the sample and some of the infrared radiation is transmitted through the sample. 3 rows Infrared IR spectroscopy is a vibrational spectroscopic technique based on the absorption of. Near Infrared NIR spectroscopy uses light transmission and absorption to measure various constituents in a sample material such as.
 Source: bruker.com
Source: bruker.com
Near Infrared NIR spectroscopy uses light transmission and absorption to measure various constituents in a sample material such as. Ad Near-infrared Spectroscopy NIR Solutions From Malvern Panalytical. Strong bonds vibrate at a higher frequency faster and heavier atoms make the bond vibrate at a lower frequency slower. The interferometer is a fundamentally different piece of equipment than the monochromator found in UVVis spectrometers. This absorption of IR photons forms the basis of IR spectroscopy.
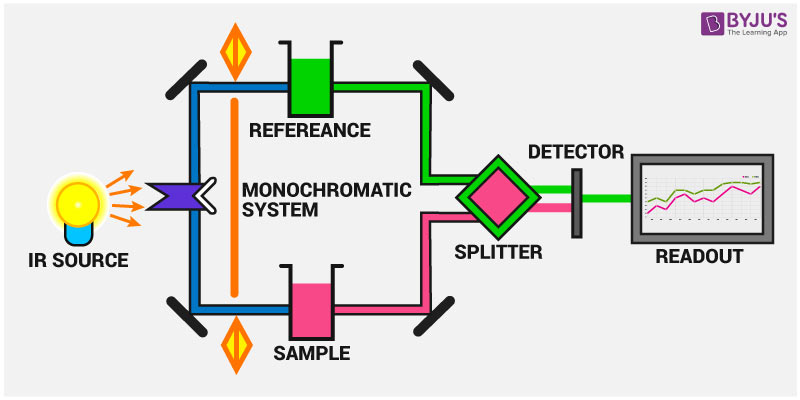 Source: byjus.com
Source: byjus.com
Infrared IR light is a wavelength of energy that is invisible to the human eye. Lower wavelengths or near infrared closest to the visible light color red are not hot and are often used to. How does FTIR Spectroscopy Work. In the interferometer the light passes through a beam splitter which sends the. Infrared IR sometimes called infrared light is electromagnetic radiation EMR with wavelengths longer than those of visible lightIt is therefore invisible to the human eye.
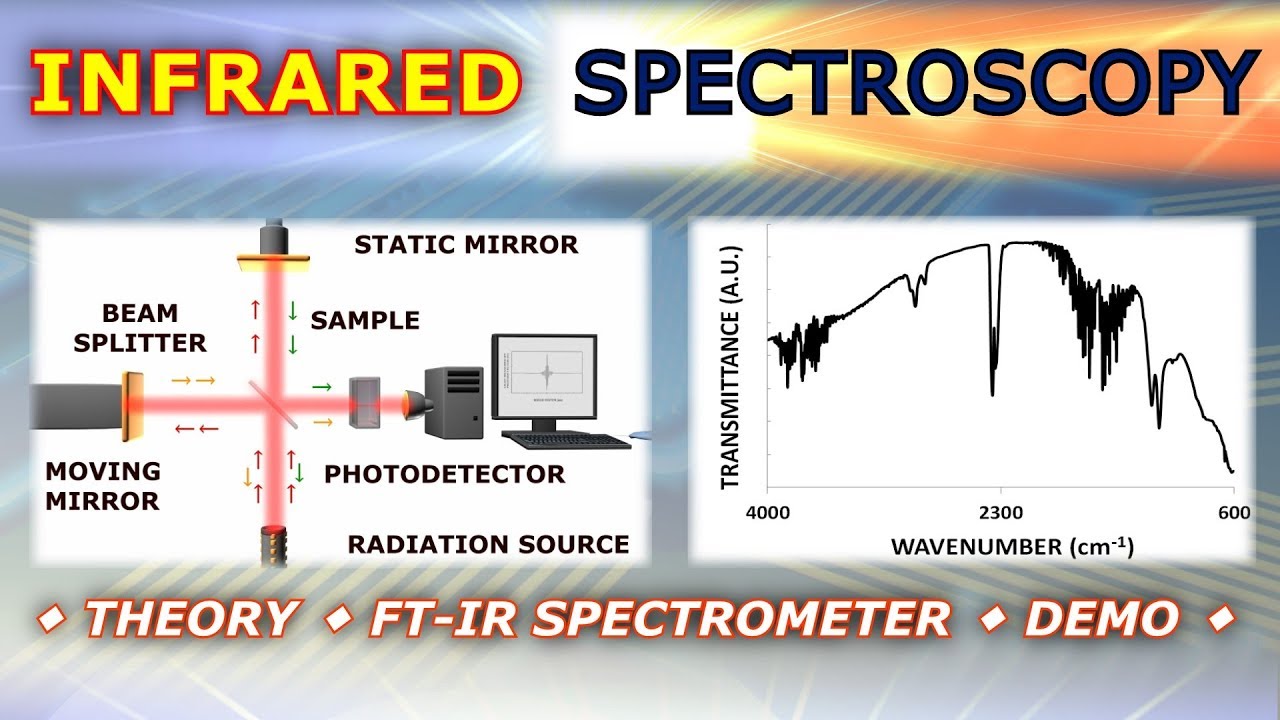 Source: youtube.com
Source: youtube.com
Near Infrared NIR spectroscopy uses light transmission and absorption to measure various constituents in a sample material such as. This can be analyzed in three ways by measuring absorption emission and reflection. Using a more scientific language the infrared colors of compounds come from the fact that certain molecular bonds absorb specific wavelengths of infrared light. Ad Near-infrared Spectroscopy NIR Solutions From Malvern Panalytical. It is a powerful method for investigating structural functional and compositional changes in biomolecules cells and tissues.
 Source: bruker.com
Source: bruker.com
Frequency or wavelength on the X-axis. IR Spectroscopy detects frequencies of infrared light that are absorbed by a molecule. Infrared IR light is a wavelength of energy that is invisible to the human eye. Whereas in dispersive IR spectroscopy monochromatic light. Different types of bonds respond to the IR radiation differently.
 Source: bruker.com
Source: bruker.com
Provides information about molecular mass and atom connectivity. Objects can have their relative temperatures measured by how much of this energy they give off. How does FTIR Spectroscopy Work. Infrared spectroscopy is widely used by chemists to determine the functional groups in molecules. Infrared spectrometers sometimes referred to as IR spectrometers measure vibrations in the interatomic bonds within the sample being tested.
 Source: pinterest.com
Source: pinterest.com
Infrared spectroscopy is widely used by chemists to determine the functional groups in molecules. It is used by chemists to determine functional groups in molecules. What is IR Spectroscopy. This spectrometer can also measure the number of absorbing molecules. Infra-red Spectroscopy Infra-red spectroscopy is used by organic chemists to help them identify compounds.
 Source: slideshare.net
Source: slideshare.net
Molecules tend to absorb these. Infrared IR spectroscopy is a vibrational spectroscopic technique based on the absorption of infrared radiation by matters that excite vibrations of molecular bonds. This makes infrared spectroscopy useful to identify functional groups in a molecule. It is a powerful method for investigating structural functional and compositional changes in biomolecules cells and tissues. In the interferometer the light passes through a beam splitter which sends the.
 Source: pinterest.com
Source: pinterest.com
The NIR instrument measures the proportion of light which is reflected by the. Basic schematic of Michelson interferometer courtesy of Leng Materials Characterization The most important component of FTIR spectroscopy and where it derives its distinction from typical infrared spectroscopy is the Michelson interferometer. Infrared IR light is a wavelength of energy that is invisible to the human eye. In infrared spectroscopy analysis infrared radiation is transmitted through a sample. Infrared IR spectroscopy is a vibrational spectroscopic technique based on the absorption of infrared radiation by matters that excite vibrations of molecular bonds.
 Source: co.pinterest.com
Source: co.pinterest.com
Provides mostly information about the presence or absence of certain functional groups. Objects can have their relative temperatures measured by how much of this energy they give off. Whereas in dispersive IR spectroscopy monochromatic light. What is IR Spectroscopy. This makes infrared spectroscopy useful to identify functional groups in a molecule.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how does ir spectroscopy work by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






