Your How does a battery work for kids images are ready in this website. How does a battery work for kids are a topic that is being searched for and liked by netizens now. You can Find and Download the How does a battery work for kids files here. Get all royalty-free vectors.
If you’re searching for how does a battery work for kids pictures information related to the how does a battery work for kids keyword, you have visit the right blog. Our website frequently gives you suggestions for seeking the maximum quality video and image content, please kindly search and locate more informative video content and graphics that match your interests.
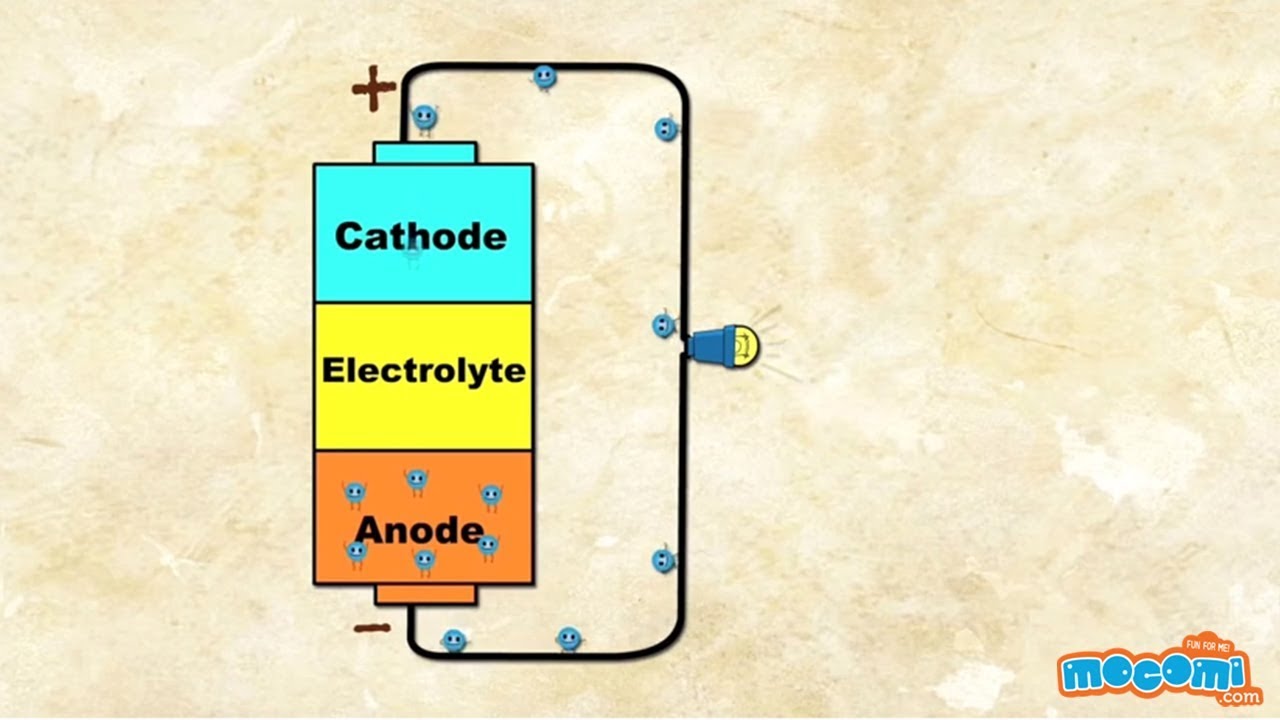
How Does A Battery Work For Kids. Most videos will tell you things like anode cathode and end up bogged down in confusion. Batteries provide portable convenient sources of energy for powering devices without wires or cables. Like a heart pumping blood around the body a battery uses the energy stored in chemicals to push electric currents around a circuit. Batteries have three parts an anode - a cathode and the electrolyte.
 Electricity For Kids And Everyone Else A Simple Introduction Electrical Engineering Humor Circuits Science Material Science From pinterest.com
Electricity For Kids And Everyone Else A Simple Introduction Electrical Engineering Humor Circuits Science Material Science From pinterest.com
There are a lot of different kinds of batteries but they all function based on the same underlying concept. How do Batteries WorkHow do batteries workYou use them to power a range of appliances at home. In most basic terms a battery cell is made up of three components. Information recall - remember the details you picked up about putting batteries into objects. Chemicals inside the battery store the energy. The chemical reactions in a battery involve the flow of electrons from one material electrode to another through an external circuit.
It basically converts stored chemical energy into electrical energy.
All batteries contain two electrodes that allow electricity to pass through them and a chemical liquid or paste called an electrolyte. During discharge the battery functions as a galvanic cell where chemical energy is converted into electrical energy. The voltage of a battery is a measure of how hard this pushing force is. What if you could learn how a battery wo. How does a battery work. A battery is a device that is able to store electrical energy in the form of chemical energy and convert that energy into electricity says.
 Source: pinterest.com
Source: pinterest.com
A battery is essentially a can full of chemicals that produce electrons. The stored chemical energy in the battery converts to electrical energy which travels out of the battery and into the base of the flashlights bulb causing it to. A battery is essentially a can full of chemicals that produce electrons. This results in an electrical difference between the anode and the cathode. 1700s animation batteries copper electricity frogs history how things work inventions metal TED Ed TKSST is an unprecedented collection of 5000 kid-friendly videos curated for teachers and parents who want to share smarter more meaningful media in the classroom and at home.
 Source: pinterest.com
Source: pinterest.com
Your watch laptop and laser-pointer are all powered by the same thing. If you look at any battery youll notice that it has two terminals. The metal parts are called electrodes. Chemistry By Mary Bates. The chemical reactions in the battery causes a build up of electrons at the anode.
 Source: pinterest.com
Source: pinterest.com
During discharge the battery functions as a galvanic cell where chemical energy is converted into electrical energy. Strictly speaking a battery consists of two or more cells connected in series or parallel but the term is generally used for a single cell. How does a battery work. A dry cell is a common type of battery used today. Batteries have three parts an anode - a cathode and the electrolyte.
 Source: pinterest.com
Source: pinterest.com
The discharge process in a. There are a lot of different kinds of batteries but they all function based on the same underlying concept. Batteries have three parts an anode - a cathode and the electrolyte. The discharge process in a. You also find them in many of.
 Source: pinterest.com
Source: pinterest.com
Today well meet a battery and hell tell us how a battery works and i. In most basic terms a battery cell is made up of three components. Batteries provide portable convenient sources of energy for powering devices without wires or cables. Chemical reactions that produce electrons are called electrochemical reactions. It is an electrochemical battery that converts the chemical energy between the two metal probes or electrodes to electrical energy by immediate transfer of electrons.
 Source: pinterest.com
Source: pinterest.com
The flow of electrons provides an electric current that can be used to do work. This results in an electrical difference between the anode and the cathode. The voltage of a battery is a measure of how hard this pushing force is. Batteries have three parts an anode - a cathode and the electrolyte. You also find them in many of your.
 Source: ar.pinterest.com
Source: ar.pinterest.com
When the battery is used the chemical energy changes into electric energy. Batteries are small things that provide portable electricity. As a battery generates power the chemicals inside it are gradually converted into different chemicals. Voltaic Pile If you want to learn about the electrochemical reactions used to create batteries it is easy to do experiments at home to try out different combinations. All batteries contain two electrodes that allow electricity to pass through them and a chemical liquid or paste called an electrolyte.
 Source: pinterest.com
Source: pinterest.com
How does a battery work. Batteries are small things that provide portable electricity. During discharge the battery functions as a galvanic cell where chemical energy is converted into electrical energy. Voltaic Pile If you want to learn about the electrochemical reactions used to create batteries it is easy to do experiments at home to try out different combinations. When the battery is used the chemical energy changes into electric energy.
 Source: pinterest.com
Source: pinterest.com
It basically converts stored chemical energy into electrical energy. It basically converts stored chemical energy into electrical energy. The potato battery explanation can be given by the presence of starch juices in potatoes along with the electrodes which helps the potato to act as a battery. How Batteries Work Inside a battery there are two pieces of metal in a liquid or a paste. But how do they work.
 Source: pinterest.com
Source: pinterest.com
Obviously this arrangement does not work very well in a flashlight but it works fine for stationary applications. It is an electrochemical battery that converts the chemical energy between the two metal probes or electrodes to electrical energy by immediate transfer of electrons. Chemical reactions that produce electrons are called electrochemical reactions. Like a heart pumping blood around the body a battery uses the energy stored in chemicals to push electric currents around a circuit. But how do they work.
 Source: br.pinterest.com
Source: br.pinterest.com
Their ability to generate power dwindles the batterys voltage slowly falls and the battery eventually runs flat. Reading comprehension - make sure that. A dry cell is a common type of battery used today. You also find them in many of your. You also find them in many of.
 Source: pinterest.com
Source: pinterest.com
The chemical reactions in a battery involve the flow of electrons from one material electrode to another through an external circuit. Chemicals inside the battery store the energy. 1700s animation batteries copper electricity frogs history how things work inventions metal TED Ed TKSST is an unprecedented collection of 5000 kid-friendly videos curated for teachers and parents who want to share smarter more meaningful media in the classroom and at home. The chemical reactions in a battery involve the flow of electrons from one material electrode to another through an external circuit. In most basic terms a battery cell is made up of three components.
 Source: pinterest.com
Source: pinterest.com
In most basic terms a battery cell is made up of three components. In most basic terms a battery cell is made up of three components. How do Batteries WorkHow do batteries workYou use them to power a range of appliances at home. Most videos will tell you things like anode cathode and end up bogged down in confusion. The chemical reactions in a battery involve the flow of electrons from one material electrode to another through an external circuit.
 Source: pinterest.com
Source: pinterest.com
Their ability to generate power dwindles the batterys voltage slowly falls and the battery eventually runs flat. An anode negative charge. The flow of electrons provides an electric current that can be used to do work. A dry cell is a common type of battery used today. A battery is essentially a can full of chemicals that produce electrons.
 Source: pinterest.com
Source: pinterest.com
Their ability to generate power dwindles the batterys voltage slowly falls and the battery eventually runs flat. As a battery generates power the chemicals inside it are gradually converted into different chemicals. Today well meet a battery and hell tell us how a battery works and i. How does a battery work. During discharge the battery functions as a galvanic cell where chemical energy is converted into electrical energy.
 Source: pinterest.com
Source: pinterest.com
There are a lot of different kinds of batteries but they all function based on the same underlying concept. The metal parts are called electrodes. Chemistry By Mary Bates. Information recall - remember the details you picked up about putting batteries into objects. How do batteries workYou use them to power a range of appliances at home.
 Source: pinterest.com
Source: pinterest.com
The flow of electrons provides an electric current that can be used to do work. Chemical reactions that produce electrons are called electrochemical reactions. Like a heart pumping blood around the body a battery uses the energy stored in chemicals to push electric currents around a circuit. The cathode and anode the positive and negative sides at either end of a traditional battery are hooked up to an electrical circuit. If you look at any battery youll notice that it has two terminals.
 Source: pinterest.com
Source: pinterest.com
A battery is a device that is able to store electrical energy in the form of chemical energy and convert that energy into electricity says. Chemical reactions that produce electrons are called electrochemical reactions. In this fun simple video learn what current really is why some materials conduct and some dont the importance of free electro. The metal parts are called electrodes. Most videos will tell you things like anode cathode and end up bogged down in confusion.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how does a battery work for kids by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






