Your How do emulsifiers work images are available. How do emulsifiers work are a topic that is being searched for and liked by netizens today. You can Find and Download the How do emulsifiers work files here. Get all royalty-free photos.
If you’re searching for how do emulsifiers work pictures information linked to the how do emulsifiers work interest, you have come to the ideal blog. Our site frequently gives you hints for seeing the maximum quality video and picture content, please kindly hunt and find more informative video content and images that fit your interests.
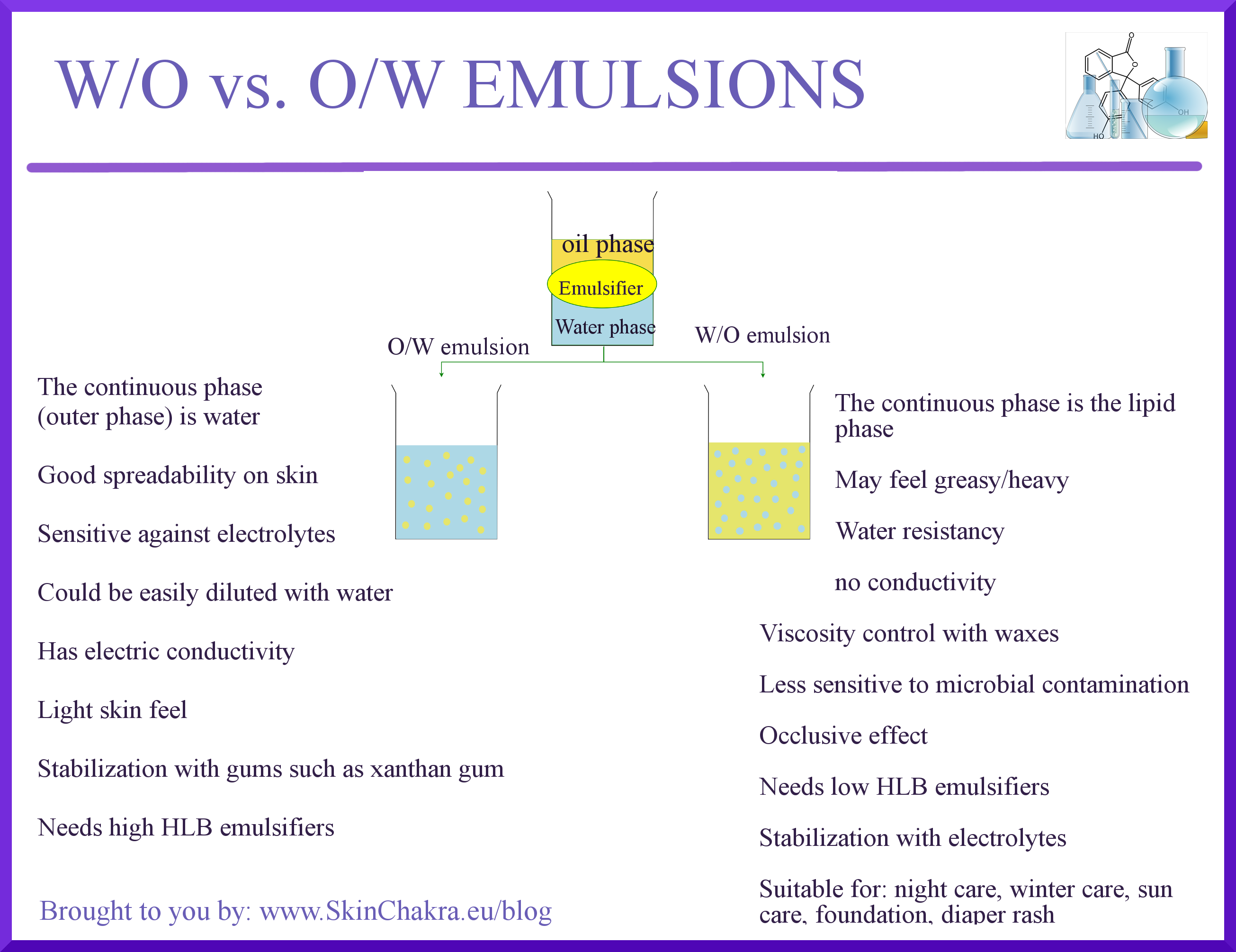
How Do Emulsifiers Work. The desired effect of an. Emulsifiers work by forming physical barriers that keep droplets from coalescing. In this case you need to choose a. Johns leaves active ingredient or ingredients whatever that or those may be.
 How Is Soap Made Today Soap Making Tallow Soap Soap From pinterest.com
How Is Soap Made Today Soap Making Tallow Soap Soap From pinterest.com
The essence of baking is chemistry and anyone who wants to be a master pastry chef must understand the principles and science that make baking work. Detergents for example enable water molecules to spread more easily allowing it to clean dirt from the surface. The res ults of the studies of the compositi on and effect. As you said emulsifiers are material that has both hydrophobic and hydrophobic groups. See below for a simplified illustration. Gellan gum which is produced by a naturally occurring microorganism is commonly used as a gelling agent.
In general emulsifiers do not work by lowering the surface tension.
Guar gum can be used to emulsify thicken and stabilize ingredients in food products even those that require cold. An emulsifier molecule works by having two parts. A type of surfactant see Sidebar emulsifiers contain both a hydrophilic water-loving or polar head group and a hydrophobic oil-loving or nonpolar tail. How do emulsifiers work. How Do Emulsifiers Work. Aside from choices of base fluid material they use a mystifying array of additives.
 Source: pinterest.com
Source: pinterest.com
Emulsifiers work by forming physical barriers that keep droplets from coalescing. How do emulsifiers work. An emulsifier is a product which enables two or more immiscible ingredients to mix. A type of surfactant see Sidebar emulsifiers contain both a hydrophilic water-loving or polar head group and a hydrophobic oil-loving or nonpolar tail. They sit between the water and oil layer bringing them together to prevent separation eggs act as emulsifiers in mayonnaise.
 Source: pinterest.com
Source: pinterest.com
This consists of three things good chemistry in the form. Johns leaves active ingredient or ingredients whatever that or those may be. Oil and water dont mixexcept when they do. The analysis of emulsifiers and t heir mixtures used in manufacturing. The water attaches to the hydrophilic or water loving head of the emulsifier while the oil attaches to the lipophilic or fat loving tail.
 Source: pinterest.com
Source: pinterest.com
Emulsifier in foods any of numerous chemical additives that encourage the suspension of one liquid in another as in the mixture of oil and water in margarine shortening ice cream and salad dressing. In the case of. What do you mean by emulsifiers. How do emulsifiers work. An emulsifier is a product which enables two or more immiscible ingredients to mix.
 Source: pinterest.com
Source: pinterest.com
An up-to-date comprehensive guide to understanding and applying food science to the bakeshop. Aside from choices of base fluid material they use a mystifying array of additives. They do lower the surface tension but it is still thermodynamically favourable for droplets to coalesce. An emulsifier molecule works by having two parts. Emulsifying agents are soluble in both oil and water accomplishing this by having one end of their molecule attracted to water hydrophilic and the other end attracted to fatsoils lipophilic.
 Source: pinterest.com
Source: pinterest.com
Guar gum can be used to emulsify thicken and stabilize ingredients in food products even those that require cold. Aside from choices of base fluid material they use a mystifying array of additives. In an emulsion the molecules will arrange there hydrophibic sides into the fat and their hydrophilic sides in the water. The desired effect of an. So what exactly is emulsification and how does the process happen in the kitchen.
 Source: pinterest.com
Source: pinterest.com
Oil and water want to hang out together as much as the headbangers and preppies did back in my day so if we want to make a lotion we need to create optimal conditions to make it work. They contain molecules that have one end that is attracted to water and the other end attracted to oil hydrophilic and lipophilic. When an emulsifier is added to an oil and water product one end of the molecule dissolves in water and the other in oil. Please give a like and leave a comment i. One part of the molecule has an electric charge like an ion and will dissolve in water but not in oil.
 Source: pinterest.com
Source: pinterest.com
How do chemists balance all these different issues. An emulsifier is a product which enables two or more immiscible ingredients to mix. They sit between the water and oil layer bringing them together to prevent separation eggs act as emulsifiers in mayonnaise. Guar gum can be used to emulsify thicken and stabilize ingredients in food products even those that require cold. Emulsifiers help prevent coalescence by forming a physical barrier between the dispersed phase and the dispersion medium.
 Source: pinterest.com
Source: pinterest.com
Emulsifiers help prevent coalescence by forming a physical barrier between the dispersed phase and the dispersion medium. What do you mean by emulsifiers. Given the above Im sure youve already guessed that the emulsifier creates a kinetic barrier. Emulsifying agents are soluble in both oil and water accomplishing this by having one end of their molecule attracted to water hydrophilic and the other end attracted to fatsoils lipophilic. They do lower the surface tension but it is still thermodynamically favourable for droplets to coalesce.
 Source: pinterest.com
Source: pinterest.com
This consists of three things good chemistry in the form. In an emulsion the molecules will arrange there hydrophibic sides into the fat and their hydrophilic sides in the water. Emulsifiers work by forming physical barriers that keep droplets from coalescing. And lets assume that it worked I am just making this up as an example. The hydrophilic end of the emulsifier molecule is attracted to the water and the hydrophobic end is attracted to the fatoil.
 Source: pinterest.com
Source: pinterest.com
Emulsifier molecules work by having a hydrophilic end water-loving and hydrophobic end water-hating. World of lubricant additives Speaking as a chemist whether it be grease or oil but especially with a MWF these issues pose a formidable set of interrelated and interacting problems. How do chemists balance all these different issues. Oil and water dont mixexcept when they do. One part of the molecule has an electric charge like an ion and will dissolve in water but not in oil.
 Source: pinterest.com
Source: pinterest.com
Given the above Im sure youve already guessed that the emulsifier creates a kinetic barrier. The res ults of the studies of the compositi on and effect. Amphiphilic molecules have parts of them that prefer to sit in the water phase hydrophilic and parts that prefer to sit in the fatty phase hydrophobic. In this case you need to choose a. DMG-75 produced the biggest bubbles.
 Source: pinterest.com
Source: pinterest.com
In this case you need to choose a. Emulsifiers also help foods like cookies and crackers maintain a light tender texture by keeping oil and water bound together so the fat is uniformly distributed. How are natural emulsifiers used in foods and beverages. An emulsifier molecule works by having two parts. How do chemists balance all these different issues.
 Source: pinterest.com
Source: pinterest.com
In this case you need to choose a. Emulsifiers increase the number of bubbles incorporated during mixing observing higher number of bubbles particularly with PGEF. Coalescence is a process in which similar particles in an emulsion come together to form larger and larger particles resulting in separation of the dispersed phase and the dispersion medium. Emulsifiers work by forming physical barriers that keep droplets from coalescing. What do you mean by emulsifiers.
 Source: pinterest.com
Source: pinterest.com
Thus these water and oil molecules are. Emulsifying agents are soluble in both oil and water accomplishing this by having one end of their molecule attracted to water hydrophilic and the other end attracted to fatsoils lipophilic. How Do Emulsifiers Work. And lets assume that it worked I am just making this up as an example. Therefore emulsifiers are attracted to both polar and nonpolar compounds.
 Source: pinterest.com
Source: pinterest.com
Emulsifier in foods any of numerous chemical additives that encourage the suspension of one liquid in another as in the mixture of oil and water in margarine shortening ice cream and salad dressing. Emulsifiers increase the number of bubbles incorporated during mixing observing higher number of bubbles particularly with PGEF. A type of surfactant see Sidebar emulsifiers contain both a hydrophilic water-loving or polar head group and a hydrophobic oil-loving or nonpolar tail. What the heck are emulsifiers and why are they necessary. This part of the molecule known as the head is called hydrophilic which means water loving.
 Source: in.pinterest.com
Source: in.pinterest.com
They do lower the surface tension but it is still thermodynamically favourable for droplets to coalesce. Johns Wort leaves in some olive oil so infuse the olive oil with the St. Suppose you have to make oil-in-water emulsion which you want to stabilize. In general emulsifiers do not work by lowering the surface tension. An emulsifier molecule works by having two parts.
 Source: pinterest.com
Source: pinterest.com
Emulsifiers-How Do They Work. What the heck are emulsifiers and why are they necessary. An emulsifier molecule works by having two parts. What do you mean by emulsifiers. Johns leaves active ingredient or ingredients whatever that or those may be.
 Source: pinterest.com
Source: pinterest.com
Emulsifiers increase the number of bubbles incorporated during mixing observing higher number of bubbles particularly with PGEF. In the case of. One part of the molecule has an electric charge like an ion and will dissolve in water but not in oil. DMG-75 produced the biggest bubbles. By vigorously mixing the emulsifier with the water and fatoil a stable emulsion can be made.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how do emulsifiers work by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






